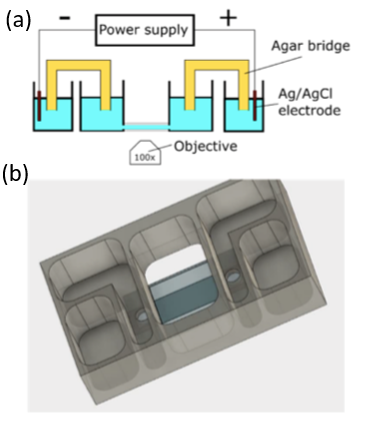
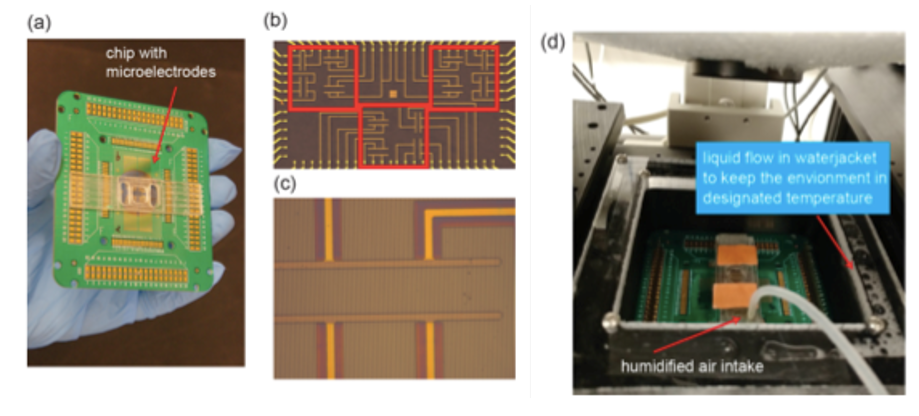
Our interdisciplinary MURI team (physics, chemistry, neuroscience, cell biology and dermatology) discovered that Biochemical and Mechanical Systems in cells are also excitable, complementing the faster and larger scale excitable systems character of membrane potentials in neurons.
Excitable systems have powerful capabilities, including the ability to exhibit sudden bursts, self-sustaining intermittent or rhythmic dynamics, and collective dynamics organized in space and time.
Notably excitable systems can store and transmit information in their dynamics in both space and time across multiple scales.
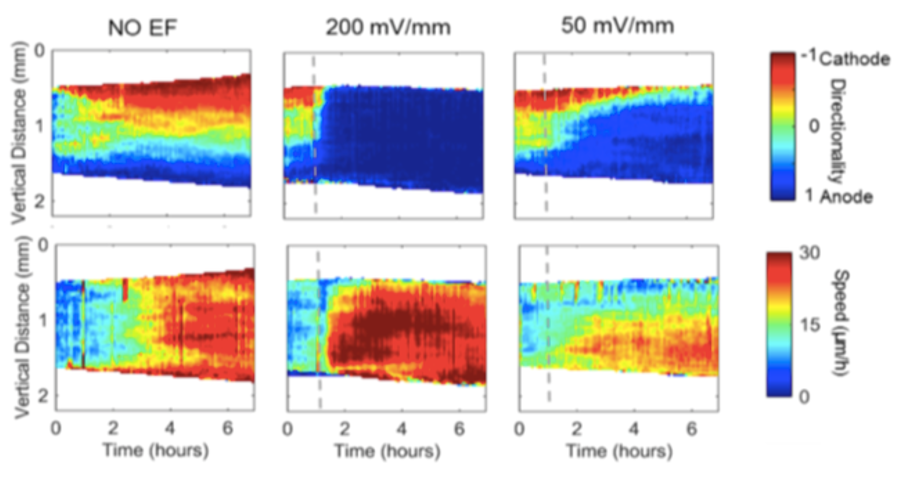
We discovered that the biochemical and biomechanical excitable systems can be precisely modulated and actuated with new synthetic biology switches, through the nanotopography of the environment, and with electric fields (EF).
This broad range of actuators exert precise control over living systems, e.g. selective activation of intracellular signaling pathways, cell migration, gene expression, and neuronal activity.